Questions 1-2
A) Hydrogen Bonding
C) Ionic Bonding E) London Dispersion Forces
B) Hybridization
D) Resonance
1. (3 pts) Is used to explain why iodine molecules are held together in the solid state.
2. (3 pts) Is used to explain why the boiling point of HF is greater than the boiling point of HBr.
3. (3 pts) Which of the following is true at the triple point of a pure substance?
A) The vapor pressure of the solid
phase always equals the vapor pressure of the liquid phase.
B) The temperature is always 0.01
K lower than the normal melting point.
C) The liquid and gas phases of
the substance always have the same density and are therefore
indistinguishable.
D) The solid phase always melts
if the pressure increases at constant temperature.
E) The liquid phase always vaporizes
if the pressure increases at constant temperature.
4. I. Difference in temperature between freezing
point of solvent and freezing point of solution
II. Molal freezing point depression
constant, Kf, for solvent
(3 pts) In addition to the information
above, which of the following gives the minimum data required to
determine the molecular mass of a nonionic
substance by the freezing point depression technique?
A) No further information is necessary.
C) Mass of solute and mass of solvent.
B) Mass of solute.
D) Mass of solute and volume of solvent
E) Mass of solute, mass of solvent, and vapor
pressure of solvent
5. (4 pts) A solution of toluene (molecular weight
92.1) in benzene (molecular weight 78.1) is prepared. The mole
fraction of toluene in the solution is 0.100.
What is the molality of the solution?
A) 0.100 m B) 0.703 m C) 0.921 m D) 1.28 m E) 1.42 m
Questions 6-8

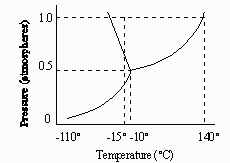
6. (3 pts) The normal boiling point of the substance
represented by the phase
diagram above is
A) -15C C) 140C
B) -10C D) greater
than 140C
E) not determinable from the diagram
7. (3 pts) The phase diagram above provides sufficient
information for
determining the
A) entropy change on vaporization
D) latent heat of vaporization
B) conditions necessary for sublimation
E) latent heat of fusion
C) deviations from ideal gas behavior
of the gas phase
8. (3 pts) For the substance represented in the diagram,
which of the phases is most dense and which is least dense
at -15C?
Most Dense
Least Dense
A) Solid
Gas
B) Solid
Liquid
C) Liquid
Solid
D) Liquid
Gas
E) The diagram gives no information
about densities.
Free Response
9. (6 pts) The freezing points and electrical conductivities
of three aqueous solutions are given below.
| Solution | Freezing Point | Electrical conductivity |
| 0.010 m sucrose | -0.0186 C | almost zero |
| 0.010 m formic acid | -0.0213 C | low |
| 0.010 m sodium fomate | -0.0361 C | high |
Explain the relationship between the freezing
point and electrical conductivity for each of the solutions above.
Account for the differences in the freezing
points among the three solutions.
10. Elemental analysis of an unknown pure substance indicates that the percent composition by mass is as follows: Carbon, 49.02%; Hydrogen, 2.743%; Chlorine, 48.23%
A solution that is prepared by dissolving 3.150
grams of the substance in 25.00 grams of benzene, C6H6, has a
freezing point of 1.12 C. (The normal
freezing point of benzene is 5.50 C and the molal freezing-point
depression constant, Kf, for benzene is 5.12
C/molal.)
A) (3 pts) Determine the
empirical formula of the unknown substance.
B) (5 pts) Using the data
gathered from the freezing point depression method, calculate the molare
mass of the unknown substance.
C) (3 pts) Calculate the
mole fraction of benzene in the solution described above.
D) (3 pts) The vapor pressure
of pure benzene at 35øC is 150. millimeters of Hg. Calculate
the vapor
pressure of
benzene over the solution described above at 35øC.
11. Discuss the following phenomena in terms of the chemical and
physical properties of the substances involved
and general principles
of chemical and physical change.
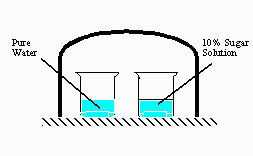
A) (5 pts) As the system
shown on the right approaches
equilibrium,
what change occurs to the volume of water in the
beaker with
pure water? What happens to the concentration
of the sugar
solution in the other beaker? Explain why these
changes occur.
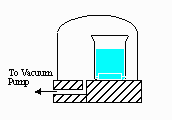
B) (5 pts) A bell jar connected
to a vacuum pump is shown on the right. As the
air pressure
under the bell jar decreases, what behavior of water in the beaker
will be observed?
Explain why this occurs.
12. Explain each of the following in terms of atomic and molecular
structures and/or
intermolecular forces.
A) (3 pts) The normal boiling point of CCl4 is 77 C, whereas that of CBr4 is 190 C.
B) (3 pts) NaI(s) is very
soluble in water, whereas I2(s) has a solubility of only 0.03 gram per
100 grams of
water.
Freebie: (3 pts) If you had your choice of any career what would it be?
1. E
2. A
3. A
4. C
5. E
6. C
7. B
8. D
9. Sucrose is a covalent compound and does not split up in water.
Therefore there are no ions presents so the electrical conductivity is
low, and there are relatively few particles present so the freezing point
depression is not as great.
Formic acid and sodium formate are both electrolytes, but judging from their electrical conductivity, the formic acid must not dissociate 100% (or at least not as much as the sodium formate). Therefore, there are fewer particles present and it has less of a freezing point depression.
10. A. C3H2Cl
B. 147.3 g/mol
C. .937
D. 140.6 mmHg
11. A. The pure water level will decrease because it has a higher vapor pressure when compared to the sugar solution. Eventually, all of the water will be transferred into the sugar solution.
B. As the pressure drops, the boiling point of the water will drop. If the water is warm enough, it will begin to boil.
12. A. Both compounds are non polar, therefore both are affected only by London Dispersion Forces. LDF's are stronger with bigger atoms, so CBr4 has larger LDF's, therefore it is harder to separate into a gas, therefore it has a higher boiling point.
B. NaI is ionic (and therefore polar) while I2 is non polar. Water is a polar solvent. Polar solvents tend to dissolve polar solutes, thus, NaI is very soluble while I2 is not.
A teacher!!