PLEASE DON'T WRITE ON ME
(20 pts) Write the electron dot structures for each of the following
atoms/ions:
| 1. S | 2. N | 3. Mg | 4. Br | 5. Kr |
| 6. Sr+ | 7. At- | 8. Sb3- | 9. Ba+4 | 10. Cl+2 |
11. (4 pts) Helium is a noble gas even though it does not have 8 valence electrons. Explain why (be very specific).
12. (25 pts) Complete the following table:
| Element | Cl | S | Be | Rn | Cs |
| No. of Valence Electrons | |||||
| Electron Dot Structure | |||||
| No. of Electrons Lost or Gained | |||||
| Which Noble Gas Will it Look Like? | |||||
| Charge on the Ion |
13 (3 pts) Why do all of the elements in the first column of the periodic table tend to have a +1 charge in their ion form?
(6 pts) Write the formula for the compounds that would be produced from the combination of the following atoms:
14. Ca + F 15. Rb + O 16.
Mg + N
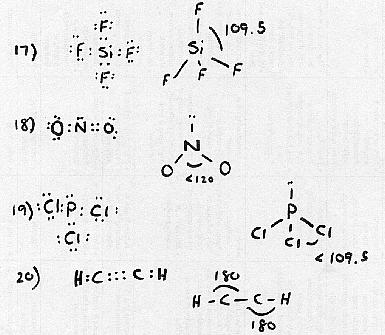
For the following compounds draw the structural formula. WARNING: To get full credit you must show all bonds (i.e. single, double, triple), all electrons, and all the angles between the atoms.
17. (3 pts) SiF4
18. (3 pts) NO2-
19. (3 pts) PCl3
20. (3 pts) C2H2 (Hint: H-C-C-H)
21. (5 pts) Describe in gruesome detail why an ionic bond forms between a metal and a non-metal. In your explanations you may include pictures and you should discuss the octet rule.
22. (4 pts) Why won't an ionic bond form between two or more non-metals? Be very specific.
23. (5 pts) Complete the structure of the following molecule showing all bonds, electrons and bond angles (NOTE: all of the electrons and bonds are not shown below. You must add them!)
Ignore this problem. There is not one like it on the test.
24. (3 pts) If your house caught on fire and you only had time to save one thing from your room before it burned down, what would it be?
For answers 1-10, the number of dots surrounding the symbol is given: