A) What data are needed to calculate the molecular mass of the liquid?
Chapter 5&6 Test
1. When a sample of oxygen gas in a closed container of constant volume is heated until its absolute temperature is doubled, which of the following is also doubled?
A) The density of the gas
B) The pressure of the gas
C) The average velocity of the gas molecules
D) The number of molecules per cm3
E) The potential energy of the molecules
2. The density of an unknown gas is 4.20 grams per liter at 3.00 atm pressure and 127 C. What is the molecular weight of this gas?
A) 14.6 B) 46.0 C) 88.0 D) 94.1 E) 138
3. Equal masses of three different ideal gases, X, Y and Z, are mixed in a sealed rigid container. If the temperature of the system remains constant, which of the following statements about the partial pressure of gas X is correct?
A) It is equal to 1/3 of the total pressure.
B) It depends on the intermolecular forces of attraction between
molecules of X, Y, and Z.
C) It depends on the relative molecular masses of X, Y, and Z.
D) It depends on the average distance traveled between molecular
collisions.
E) It can be calculated with knowledge only of the volume of
the container.
4. CH4(g) + 2 O2(g) CO2(g) + 2 H2O(l) H = -889.1 kJ
H2O(l) = -285.8 kJ/mole
CO2(g) = -393.3 kJ/mole
What is the standard heat of formation of methane, CH4(g), as calculated from the data above?
A) -210.0 kJ/mole C) -75.8 kJ/mole E)
210.0 kJ/mole
B) -107.5 kJ/mole D) 75.8 kJ/mole
5. Two flexible containers for gases are at the same temperature and pressure. One holds 0.50 grams of hydrogen and the other holds 8.0 grams of oxygen. Which of the following statements regarding these gas samples is FALSE?
A) The volume of the hydrogen container is the same as the volume
of the oxygen container.
B) The number of molecules in the hydrogen container is the same
as the number of molecules in the oxygen container.
C) The density of the hydrogen sample is less than theat of the
oxygen sample.
D) The average kinetic energy of the hydrogen molecules is the
same as the average kinetic energy of the oxygen molecules.
E) The average speed of the hydrogen molecules is the same as
the average speed of the oxygen molecules.
6. A compound is heated to produce a gas whose molecular weight is to be determined. The gas is collected by displacing water in a water-filled flask inverted in a trough of water. Which of the following is necessary to calculate the molecular weight of the gas, but does NOT need to be measured during the experiment?
A) Mass of the compound used in the experiment.
B) Temperature of the water in the trough.
C) Vapor pressure of the water.
D) Barometric pressure.
E) Volume of water displaced from the flask.
7. When the actual gas volume is greater than the volume predicted by the ideal gas law, the explanation lies in the fact that the ideal gas law does NOT include a factor for molecular
A) volume B) mass C) velocity D) attractions E) shape
8. NH3(g) + 2 CH4(g) + 5/2 O2(g) H2NCH2COOH(s) + 3 H2O(l)
At constant temperature, H, the change in enthalpy for the reaction above is approximately equal to
A) E - (11/2)RT C) E + RT E) E +
(11/2)RT
B) E - (7/2)RT D) E + (7/2)RT
9. A sample of 9.00 grams of aluminum metal is added to an excess of hydrochloric acid. The volume of hydrogen gas produced at standard temperature and pressure is
A) 22.4 L B) 11.2 L C) 7.46 L D) 5.60 L E) 3.74 L
Free Response:
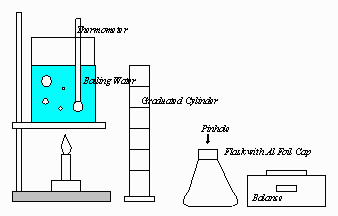
10. An experiment is to be performed to determine the molecular mass
of a volatile liquid by the vapor density method. The equipment shown
below is to be used for the experiment. A barometer is also available.

A) What data are needed to calculate the molecular mass of the
liquid?
B) What procedures are needed to obtain these data?
C) List the calculations necessary to determine the molecular mass.
D) If the volatile liquid contains nonvolatile impurities, how would the calculated value of the molecular mass be affected? Explain your reasoning.
11. Propane, C3H8, is a hydrocarbon that is commonly used as fuel for cooking.
A) Write a balanced equation for the complete combustion of propane gas, which yields CO2(g) and H2O(l).
B) Calculate the volume of air at 30 C and 1.00 atmosphere that is needed to burn completely 10.0 grams of propane. Assume that air is 21.0 percent O2 by volume.
C) The heat of combustion of propane is -2,220.1 kJ/mol. Calculate the heat of formation of propane given that the standard heat of formation of H2O(l) = -285.3 kJ/mol and the standard heat of formation of CO2(g) = -393.5 kJ/mol.
D) Assuming that all of the heat evolved in burning 30.0 grams
of propane is transferred to 8.00 kilograms of water (specific heat = 4.18
J/gK), calculate the increase in temperature of the water.
Answers
1. B
2. B
3. C
4. C
5. E
6. C
7. A
8. A
9. B
| 10. | A. |
|
| B. | See the procedure of the last lab that you did | |
| C. |
|
|
| D. | Molar mass increases |
11. A. C3H8 + 5 O2 --> 3 CO2
+ 4 H2O
B.
134 L
C.
-101.6 kJ/mol
D.
45.2 K