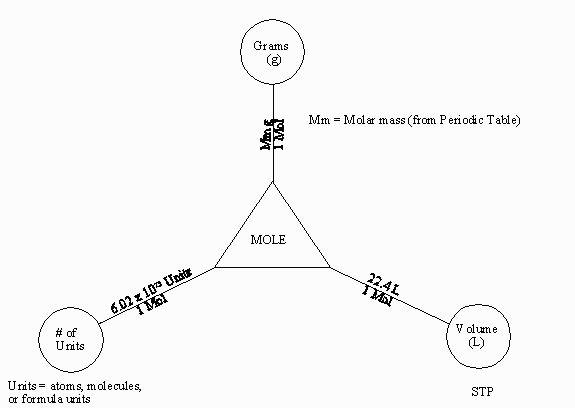
In chemistry if you are not proficient at converting between chemical
units you will have a rough time. This exercise will hopefully help
you to do these conversions easily.

After the diagram above has been explained by your teacher refer
to it while performing the following conversions:
Single-step conversions
1. 32.4 molecules Cl2 mole Cl2 (5.38 x 10-23)
2. 4.52 mole Ag atoms Ag (2.72 X 10 24)
3. 75.3 g NH3 mole NH3 (4.42)
4. 0.0453 mole CH4 g CH4 (.727)
5. 33.4 L H2 mole H2 (1.49)
6. 1.99 mole N2 L N2 (44.6)
Two-step conversions (cha cha cha)
7. 1.45 g O2 L O2 (1.02)
8. 4.00 x 1025 NaCl g NaCl (3.88 x 103)
9. 401 L NO2 molecules NO2 (1.08 x 1025)
Conversion Mania!!
10. 3.6 g CO2 atoms of O (9.9 x 1022)
11. 3.65 x 1012 NH4+ moles (NH4)2SO4 (3.03 x 10-12)
12. 54.3 g Na3PO4 moles Na+ (.993)
13. 25.7 L CO2 atoms (C & O) (2.07 x 1024)
Word problems
14. A balloon at STP is filled with 2.34 L of H2. How many
molecules of H2 are in the balloon? How many H atoms are in the balloon?
What is the mass of H2 in the balloon?
(6.29 x 1022, 1.26 x 1023, .211 g H2)
15. One hiccup has a volume at STP of approximately .20 L.
If a hiccup is made up of pure O2, what is the mass of one hiccup?
(.29 g)
16. A human tear drop has a mass of approximately .100 g.
How many molecules of H2O are contained in one tear drop?
(3.34 x 1021)
17. Given: 10.0 g C2H6
10.0 g NH3
a. Which of the above quantities contains more molecules?
b. Which of the above quantities contains more atoms?